
Types Of Copper Oxide And Its Uses
Copper is a good conductor of electricity, making it useful in many applications. However, the properties change after Oxidation. Copper Oxide is a compound that is formed after the combination of two elements, Copper and Oxygen. It can be of four types:
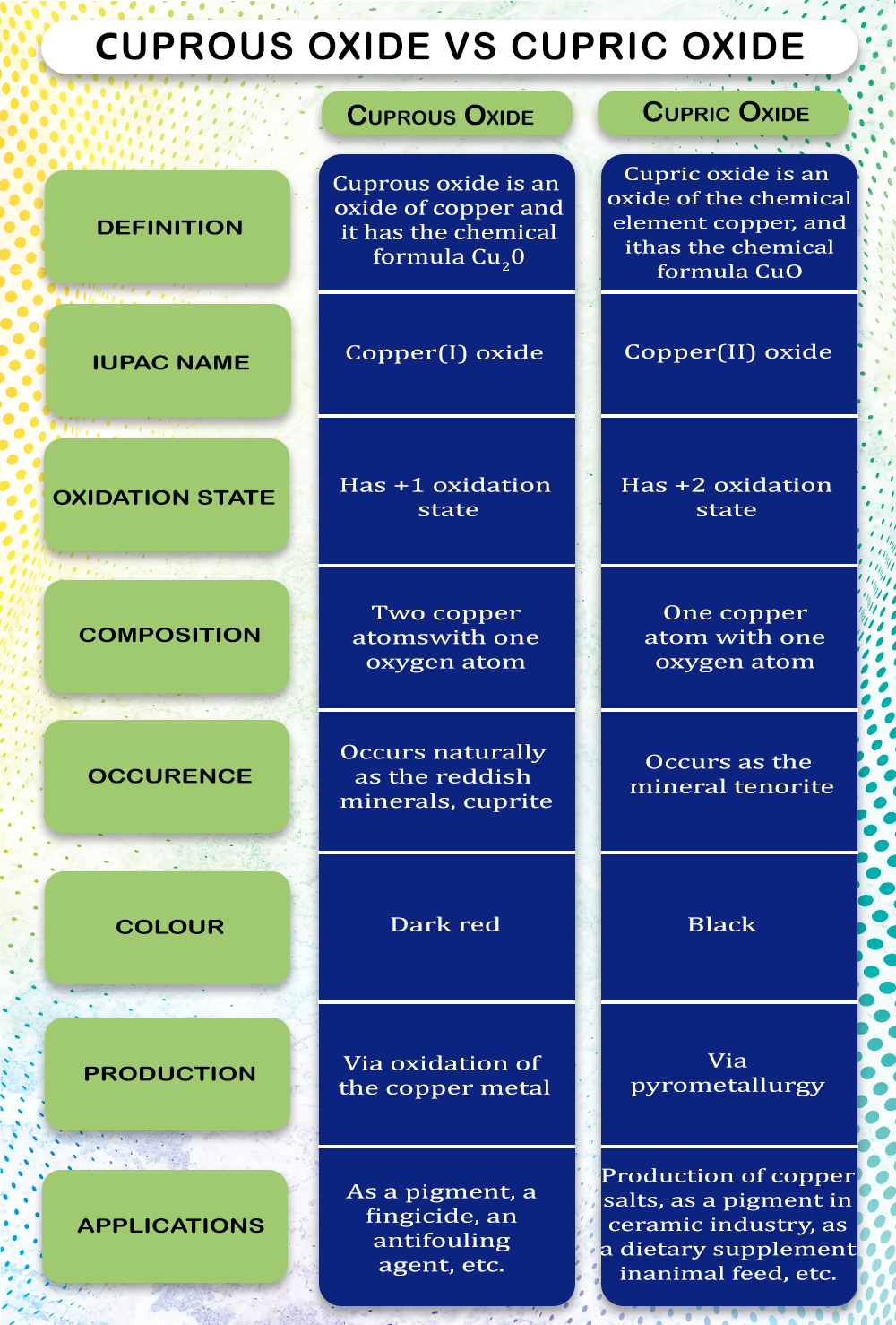
1. Copper (I) Oxide
2. Copper (II) Oxide
We can very quickly reduce the Copper Oxide back to Copper by applying heat to it. The heat can be replaced by electricity in some cases. Though we name four different types of Copper Oxide, Cupric Oxide and Cuprous oxide are the two forms commonly used for various purposes. Of the two, Cupric oxide is widely used in the fireworks industry. Cupric Oxide (Black) is a coloring agent that also helps increase the brightness of the spark in fireworks. As a firework's propellant, it logarithmically increases the oxygen content in the fireworks, ultimately increasing the brightness.
Let us get into some details about Copper Oxide uses and types.
Types of Copper Oxide:
1. Copper (I) Oxide
Copper (I) Oxide has the chemical formula Cu2O and is known as Cuprous Oxide. It is covalent and can be reduced by Hydrogen by applying heat. It is subjected to acid solution for disproportionation and produces Copper (II) Oxide. To get vice versa, the Copper (II) Oxide can react with metallic Copper in the presence of heat to produce Copper (I) Oxide. Cuprous Oxide appears in red in solid form. The reaction with the air creates a thin oxide layer that acts as a barrier against corrosion.
Uses
1. The anti-corrosion property makes it ideal for painting the bottom of the ships sailing in the sea. It is done to protect the ship's surface against the damage caused by seawater. It is also used as a component of anti-fouling paints.
2. It is used in photocells to fabricate products such as light meters and rectifiers.
3. Copper Oxide is also used in high-tech products, including semiconductors and superconductors.
4. It is also used in seed dressings.
5. It is used in many thermoelectric materials and other areas, such as glass and sensing materials.
2. Copper (II) Oxide
The compound Copper (II) Oxide with the chemical formula CuO is also called Cupric Oxide. It is an inorganic compound used as a precursor in many copper products. It is found naturally on the Earth. For synthetic preparation, Copper metal is heated in the air to get Cupric Oxide. It is mixed as a component in many over-the-counter supplements for vitamins and minerals for human consumption.
Uses
1. It is actively used as a component of the pigments used to color various products, including enamels, artificial gems, and ceramics.
2. It is also used in manufacturing insecticides and fumigants.
3. Copper (II) Oxide is used as an anti-fouling agent for painting the boat hulls.
4. Wood industry is also one of the popular application areas where Cupric Oxide is used in wood preservatives.
5. Cupric Oxide is used as a firework's propellant.
Conclusion
Any Copper Oxide will work its best in the purest form. Industries such as fireworks rely on the purity and effectiveness of Copper Oxide. As leading Cupric Oxide manufacturers, we have been using manufacturing processes per industry standards. We have been catering to the fireworks department for years with the best quality Copper Oxide (Black). We can produce Cupric Oxide in bulk. We are one of the established Cupric Oxide suppliers with an excellent manufacturing and storage facility. We have strict guidelines for storage to ensure that the compound is not exposed to air or any other environment capable of changing its chemical composition and property. Therefore, we can supplement you with the finest quality of Cupric Oxide in bulk. You can contact us to understand our production and storage process. Contact us today to get the purest quality Cupric Oxide at affordable rates to get the best results in your applications.
Frequently Asked Questions About Copper Oxide :
Copper oxides exist in two different forms: cupric oxide (CuO) and cuprous oxide (Cu2O), depending on the valence state of copper. Here, we concentrate on CuO.
Copper oxide used for various applications, such as the development of supercapacitors, near-infrared filters, in magnetic storage media, sensors, catalysis, semiconductors, etc.
Cu2O is obtained by oxidation of copper metal or reduction of copper (II) solutions with sulfur oxide, whereas CuO is obtained by pyrometallurgical processes used to extract copper from ores.
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide.
